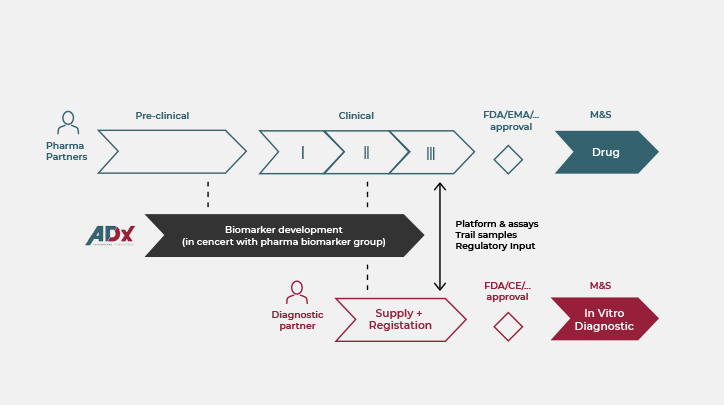
”Too often our internally developed biomarkers hit the wall when we want to use them in clinical trials. We therefore rely on biomarker development expertise at ADx to develop robust and sensitive assays for our (pre-)clinical drug development. They understand the biology of the biomarkers. We stay in the driver seat but ADx takes care of the rest.
Senior Pharma Biomarker Director
Guaranteed results
We provide the resources and expertise needed to have your neuro biomarker available in time. Our approach features strict time-lines and deliverables with clear go/no-go decision points. No surprises, step out at any time.
Access to state-of-the-art biomarkers
Get access to our extensive portfolio of state-of-the-art custom-made and fit-for-purpose assays and qualified antibodies. In addition to the research by our excellent scientific team, our extensive academic network ensures access to the latest innovative markers.
From concept to the patient
Research biomarkers are often not suited for clinical use. At ADx, we develop assays tailored to your needs taking into account the context-of-use. Whether for target engagement, or patient selection for clinical trials and in clinical practice, we provide a reliable and robust test.
During implementation in studies and trials, we can provide small scale service testing. Subsequent scale-up is made possible thanks to our partnerships with Fujirebio. Have a kit ready worldwide when your drug hits the market.
Focus on quality
Our operations are focused on guaranteeing a constant supply of qualitative materials.
- Our assays are developed in accordance with state-of-the-art industry guidance ensuring their reliability and robustness;
- We are a Certified Biobank, treating patient samples with the highest regard for ethical standards
- We are working towards ISO certification of our antibody production processes documenting our consistent quality

